A COVID-19 Shot in the Arm
Shortly after the COVID-19 pandemic broke out in early 2020, scientists and researchers around the world went to work to develop vaccines to fight SARS-CoV-2, the virus that causes COVID-19 disease. On Dec. 11, the Food and Drug Administration (FDA) issued an emergency use authorization allowing Pfizer-BioNTech’s COVID-19 vaccine to be distributed in the U.S. A week later, a second vaccine, developed by Moderna, received the same FDA emergency use authorization.
The Current spoke with Dr. Scott Grafton, M.D., UC Santa Barbara’s COVID-19 coordinator and a Distinguished Professor in the Department of Psychological & Brain Sciences, and Chuck Samuel, the Charles A. Storke Professor and Distinguished Professor Emeritus in the Department of Molecular, Cellular and Developmental Biology, about how the vaccines work, how effective they are and how scientists were able to make them available so quickly.
TC: Can you give us a lesson on how vaccines work in general?
SG: Over the course of our lives, our immune systems are constantly learning to differentiate between what is foreign to our bodies and what belongs there. Continual exposures in the gut, the lungs, sinuses, blood, skin and other organs help the immune system identify and remember viruses, bacteria, fungi and other potentially lethal entities. The immune system finds specific targets on these agents and uses them to organize a response for their elimination. For viruses, the target is often a bit of protein on their surface that the immune system can recognize.
But the learning process can take a few days or more, and each time a person becomes infected with an unfamiliar virus there is a race between the speed at which the virus is copying itself and causing symptoms and the quickness of the immune system to learn about and act against it.
A key strategy behind all vaccines is to train the immune system to recognize the offending agent without infecting the person in a way that might cause harm. This has classically been done by creating a weaker version of the virus, one that still shows off a bit of protein the immune system learns to recognize, but doesn’t grow particularly well in the person. In that way the immune system easily wins the race. These are the “live-attenuated” vaccines commonly in use.
CS: Yes, the live-attenuated vaccine strategy was the classical approach that produced some great vaccines — measles and OPV polio are good examples.
How is the COVID-19 vaccine different?
CS: The CoV-2 is not a simple virus. Coronaviruses are the largest RNA viruses that we know in terms of genetic information. Their information is contained in roughly 30,000 nucleotides. The polio virus for comparison is about 7,500 and the measles virus is about 15,000.
The COVID-19 vaccine is different from the live-attenuated vaccines for measles or polio because the COVID-19 vaccine is not the whole virus. Rather, it is a small piece of the virus’s genetic code, or genome. One of the genes of the CoV-2 virus specifies a protein called spike. Spike is on the surface of the CoV02 virus and it is the target of protective antibodies generated by our immune system.
SG: Each virus is a small bag of RNA, the molecule with code for making proteins. Once inside the body, the RNA code from the virus produces proteins that hijack the cells and enslave them to make more virus. Sticking out of this “bag” of the SARS-CoV-2 virus are bits of proteins that look like spikes. These spikes help the virus gain access to the inside of the cells that line a person’s blood vessels. The lungs, mouth and sinuses are significant points of entry.
Rather than creating an attenuated virus, the Moderna and Pfizer vaccine makers pursued an old and simple strategy that has finally come to technical fruition. They made artificial tiny bags out of fatty molecules and loaded them with the RNA code for making some of the spike. However, the bags don’t include all the other virus RNA that is required to make copies of itself. When the bags of RNA are injected into the arm, the cells in the muscle incorporate the RNA and make the spikes. The immune cells detect these spikes, recognize them as foreign and memorize their shape. The pain in the arm a person feels a day after the shot is a result of the emergence of spikes on the person’s cells and the immune system being drawn into the area and learning their shape.
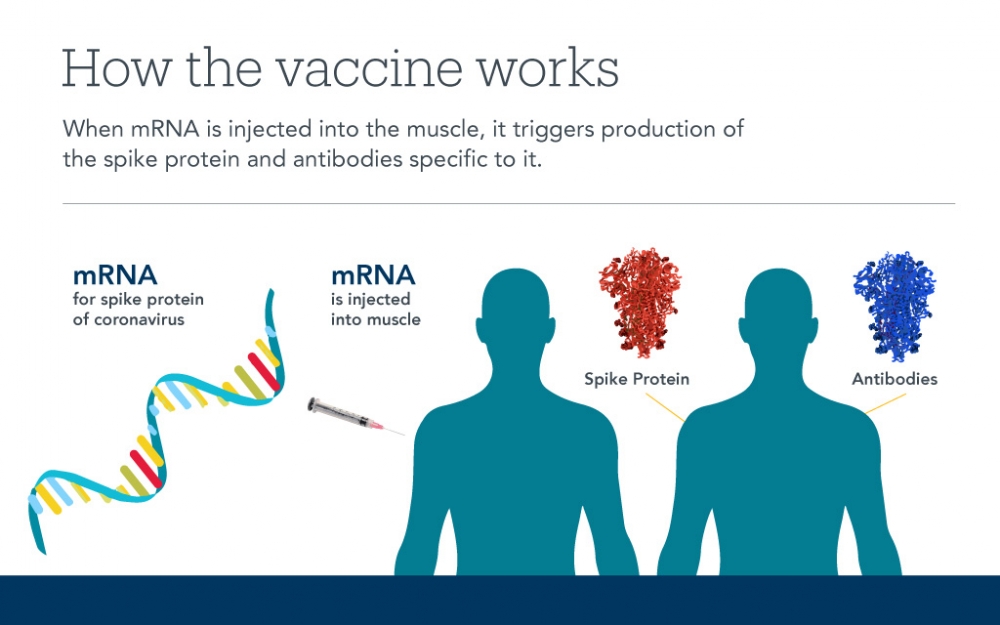
The second shot provides additional exposure of the spike to the immune system to train it further. With the memory that is formed, future exposures to the SARS-CoV-2 virus from other people will lead to a recognition by the immune system and a quick response, thereby protecting the individual. New data suggests the Moderna vaccine leads to immune memory lasting for at least a year.
The RNA vaccine is a clever approach because no virus grows in the body. The RNA injected into the muscle in the arm is a natural molecule the body can clear out over time. And, it cannot become part of a person’s DNA. There is nothing in the vaccine that can alter a person’s genetic code, nor the DNA of their ovum or sperm.
TC: Is this “new” vaccine technology?
CS: The mRNA vaccine approach is new. But the idea of a subunit, a small part of the virus as the basis of the vaccine is not new. There are two very successful vaccines that work as subunit vaccines. One is for hepatitis B — HBV — and one is for human papilloma virus — HPV. Each of these is a subunit vaccine, where a virus protein component is expressed alone and then that protein subunit is what’s used as the antigen to generate the immune response in the vaccine. It’s a component of the virus, not the whole virus. Subunit vaccines are very safe and can be very effective. That’s the conceptual strategy behind the new SARS-CoV-2 mRNA vaccines available from Moderna and Pfizer that Scott described, though our bodies make the protein from the vaccine-delivered mRNA.
How were the Pfizer and Moderna vaccines able to be developed so quickly, and how do they differ from one another?
CS: It was a year ago last week that the CoV-2 virus was identified, and a year ago this week that the RNA genome sequence was made available. In less than a year we got two mRNA vaccines that are fabulous in terms of providing protection against COVID-19. They could be developed quickly because lots of years of research by a number of laboratories laid the foundations for understanding coronaviruses, for understanding different mechanisms of gene expression and for developing the tools of molecular biology to study genes and their products. When this disease came along, all this background knowledge was mobilized in a very rapid way. It wasn’t starting from scratch. There was a good foundation of knowledge.
SG: Over the past 30 years, immunologists have learned what kinds of protein bits might be better than others at training the immune system. The technology for making lots of RNA in whatever sequence is desired has become commonplace. Once the RNA of the SARS-CoV-2 virus was sequenced in early 2020, expert choices were made as to what part of the spike would be most important for the immunes system to recognize and this determines what RNA code to package as a vaccine. The choice of what bits of the spike code to include differ with the Pfizer and Moderna vaccines, but the difference seems to be inconsequential; both lead to superb protection from SARS-CoV-2.
The other piece of technology that needed to mature for this “novel” approach to work was the fabrication of nanoscale fat bags to hold the RNA. Here, too, nanofabrication has come of age across the biosciences. The Moderna and Pfizer vaccines differ in how they keep these little fat bags held together with stabilizing molecules. To remain stable, the Pfizer fat particle must be stored at a lower temperature than the Moderna solution.
It’s natural to wonder how these vaccines appeared so quickly when, historically, vaccines can take years to develop. There is no single reason. It is really a triumph of the sciences coming together to leverage existing knowledge on how viruses work, in RNA biology, in nanofabrication and intense effort and dedication by an enormous community. With these abilities this new type of vaccine could be created very quickly from scratch.
Testing effectiveness of a vaccine for protecting people from a virus that is as widespread as SARS-CoV-2 requires many volunteers. Both the Pfizer and Moderna clinical trials compared more than 10,000 vaccine recipients to equivalent numbers of people who received a sham vaccine. For both vaccines, this was accomplished in less than five months. The results were dramatic — each was outstanding at reducing the risk of infection. The Pfizer and Moderna vaccines were effective in preventing symptomatic infections 94-95% of the time. In comparison, the seasonal flu vaccine cuts the risk by 40-60%. Data is now being collected to establish how well the vaccines block asymptomatic infection.
Given the speed at which they were developed, how do we know the vaccines are safe?
SG: Safety of vaccines in people is established in phases. First, there needs to be evidence that there are no dramatic adverse outcomes in a small number of people. In early trials, both the Moderna and Pfizer vaccines passed this hurdle with flying colors. Next, there needs to be evidence that in 15,000 to 20,000 people there aren’t an excess number of adverse events in people receiving the vaccine compared to those getting a placebo. Here, too, both vaccines readily passed.
The problem with all vaccines is that there could still be some lingering problems that occur, albeit rarely. To sort out rare events, one needs data from millions of vaccine recipients. Normally, vaccines roll out slowly, so this safety signal can take years to acquire. This set the stage for a critical decision for what to do in November 2020. Imagine you are an FDA panelist. Every day thousands of people in the nation are dying of COVID-19. Hospitals are reaching or exceeding their capacity, and more and more of your health care providers aren’t well enough to staff them. Given the effectiveness data — 95% — and the reassuring safety signals from 15,000 people, wouldn’t you give a green light to approve either of these vaccines? The FDA approved both, leaving open the door that we would subsequently learn what the very rare adverse events might be.

Photo Credit: Centers for Disease Control and Prevention
Unlike with other vaccines, the development of the SARS-CoV-2 vaccine and FDA approval for its use happened very quickly. Already millions of people have received at least one dose. Thus, the safety signals are coming in faster than with other vaccines and, so far, the news has been good. The only major concern has been with a small number of people who have developed a severe allergic reaction, called anaphylaxis. This is a reaction similar to the severe allergic response some people get to bee stings, leading them to keep an EPI pen always at the ready. Current estimates, subject to revision, suggest this occurs in 5 or 6 people per million receiving the dose or, 2 to 3 people out of everyone in Santa Barbara County.
Given these rare severe allergic reactions with the SARS-CoV-2 vaccine, it is helpful to think like a detective for a moment and ask what might be the offending ingredient in the vaccine. There are only three possibilities: the RNA, the bag of fat or the stabilizing molecules. RNA is in every cell of the body, so that can’t be the trigger. The fat molecules making the bags also are treated as friendly by the immune system, so that can’t be the trigger. The only suspect then is the stabilizing molecule. The one used by both Moderna and Pfizer is polyethylene glycol, or PEG. It’s not used in any of the other common vaccines that people receive. Instead, the more familiar vaccines use polysorbate. One current theory is that a rare individual with an allergy to polysorbate might cross react to the PEG. All vaccination sites have an EPI pen and providers who are trained to manage rare occurrences of anaphylaxis.
TC: How well do the Pfizer and Moderna vaccines protect against the SARS-CoV-2 variants that have been emerging? Is it possible variants will be identified that are beyond the scope of the Pfizer and/or Moderna vaccines?
CS: Good question. RNA virus variants can arise over time with some viruses One reason is that the viral enzyme that makes new copies of viral genome RNA occasionally makes mistakes that then can lead to mutations in the protein product. Mutations that affect the spike protein, for example, have the potential to result in altered biology. Hopefully, when our immune system generates neutralizing antibodies against a virus protein such as spike, it is a robust polyclonal response that targets multiple protein epitopes, or regions, and retains some degree of protection even against variants to reduce disease. The question of how well the Moderna and Pfizer mRNA virus vaccines recognize different variants is currently under study. Historically for some RNA virus vaccines like influenza, updates become needed; but for other viruses, like measles, that is not the case. We need to learn what will be required for COVID protection over time.
Who should not get a COVID-19 vaccine?
SG: The safety guidelines for the RNA-based vaccines can be found on the Centers for Disease Control website. If a person has a prior severe allergic — anaphylactic — reaction to other vaccines, they should seek medical advice before getting the Moderna or Pfizer vaccine. Also, if a person has a severe allergic reaction to their first Moderna or Pfizer vaccine, they should not get a second dose.
Otherwise, according to the CDC, people with food or drug allergies can receive the vaccine, as can people on any medications and those who have had COVID-19. There is no safety data yet for pregnant women or breast-fed babies.
TC: We are told that despite the vaccine it’s important for all of us to continue practicing our “COVID behavior” — wearing face coverings and staying physically distanced from people outside our households. Why is that?
CS: It’s important for a combination of reasons. One is that it likely will take longer than hoped to get enough people immunized so we achieve herd immunity, which means the greater population has immunity against the virus. And both doses are necessary at the proper interval — 21 days for Pfizer and 28 days for Moderna — to reach the fullest immunity.
Also, as Scott mentioned, we don’t know to what extent the vaccine prevents infection transmission. The trials for the Pfizer and Moderna vaccines measured their protection against disease — symptoms of COVID-19 — but I don’t know that they measured infection. Infection causes disease, but there can also be infection with no disease symptoms — hence, all those asymptomatic people. No doubt there is protection against infection, but we just don’t know yet that it’s at the 94-95% efficacy that is seen for disease. It might be less against infection. So the continued practice of good COVID social behavior, including use of face coverings and physical distancing will remain important a while longer.
What do you say to people who are hesitant about receiving a vaccine?
SG: The emergence of the RNA-based vaccines in less than 12 months is a scientific and technologic triumph. They are a gift to all of us to counteract the most devastating global disease of the past century. Recipients of the vaccine do more than protect themselves. They protect and serve their community by blunting transmission with everyone they encounter.