Seeing the Action
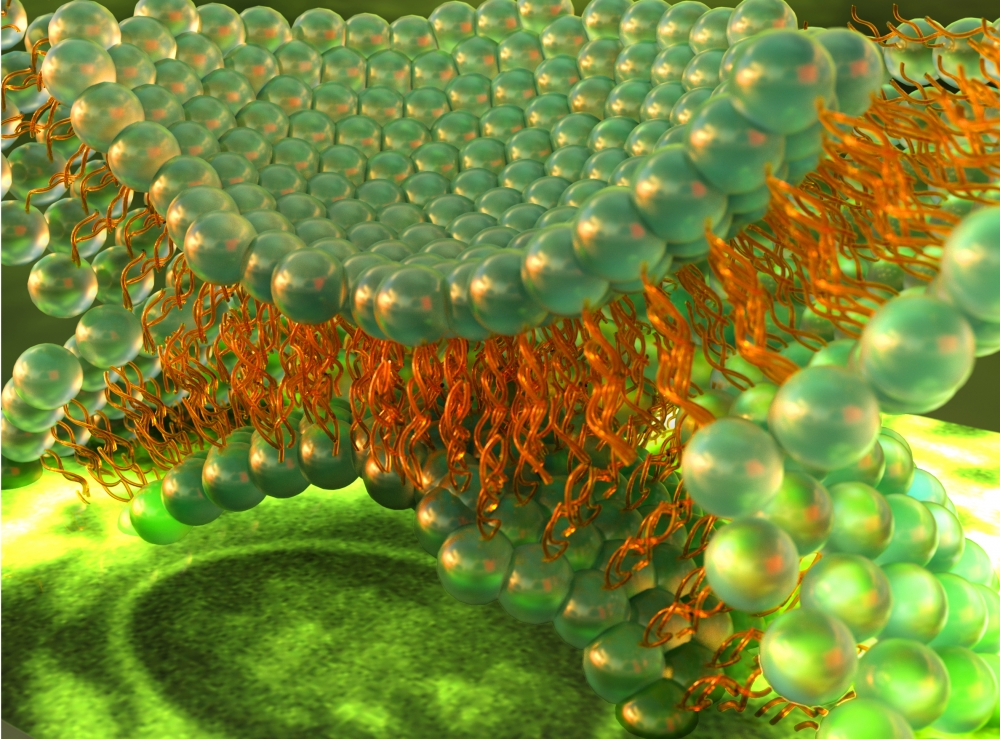
Cells are biological wonders. Throughout billions of years of existence on Earth, these tiny units of life have evolved to collaborate at the smallest levels in promoting, preserving and protecting the organism they comprise. Among these functions is the transport of lipids and other biomacromolecules between cells via membrane adhesion and fusion — processes that occur in many biological functions, including waste transport, egg fertilization and digestion.
At UC Santa Barbara, chemical engineers have developed a way to directly observe both the forces present and the behavior that occurs during cell hemifusion, a process by which only the outer layers of the lipid bilayer of cell membranes merge. While many different techniques have been used to observe membrane hemifusion, simultaneous measurements of membrane thickness and interaction forces present a greater challenge, according to Dong Woog Lee, lead author of a paper that appears in the journal Nature Communications.
“It is hard to simultaneously image hemifusion and measure membrane thickness and interaction forces due to the technical limitations,” he said.
However, by combining the capabilities of the Surface Forces Apparatus (SFA) — a device that can measure the tiny forces generated by the interaction of two surfaces at the sub-nano scale — and simultaneous imaging using a fluorescence microscope, the researchers were able to see in real time how the cell membranes rearrange in order to connect and open a fusion conduit between them. The SFA was developed in Professor Jacob Israelachvili’s Interfacial Sciences Lab at UCSB. Israelachvili is a faculty member in the Department of Chemical Engineering at UCSB.
To capture real time data on the behavior of cell membranes during hemifusion, the researchers pressed together two supported lipid bilayers on the opposing surfaces of the SFA. These bilayers consisted of lipid domains — collections of lipids that in non-fusion circumstances are organized in more or less regularly occurring or mixed arrangements within the cell membrane.
“We monitored these lipid domains to see how they reorganize and relocate during hemifusion,” said Lee. The SFA measured the forces and distances between the two membrane surfaces as they were pushed together, visualized at the Ångstrom (one tenth of a nanometer) level. Meanwhile, fluorescent imaging made it possible to see the action as the more ordered-phase (more solid) domains reorganized and allowed the more disordered-phase (more fluid) domains to concentrate at the point of contact.
“This is the first time observing fluorescent images during a hemifusion process simultaneously with how the combined thickness of the two bilayers evolve to form a single layer,” said Lee. This rearrangement of the domains, he added, lowers the amount of energy needed during the many processes that require membrane fusion. At higher pressures, according to the study, the extra energy activates faster hemifusion of the lipid layers.
Lipid domains have been seen in many biological cell membranes, and have been linked to various diseases such as multiple sclerosis, Alzheimer’s disease, and lung diseases. According to the researchers, this novel device could be used to diagnose, provide a marker for, or study dynamic transformations in situations involving lipid domains in pathological membranes. The fundamental insights provided by this device could also prove useful for other materials in which dynamic changes occur between membranes, including surfactant monolayers and bilayers, biomolecules, colloidal particles, surfactant-coated nanoparticles and smart materials.