UC Santa Barbara Study Provides a New Framework for Understanding the Energetics of Ionic Liquids
A new study by researchers at UC Santa Barbara provides clues into the understanding of the behavior of the charged molecules or particles in ionic liquids. The new framework may lead to the creation of cleaner, more sustainable, and nontoxic batteries, and other sources of chemical power. The research was published in an early online edition of the Proceedings of the National Academy of Sciences.
"I think this framework would provide a nice strategy to begin discussions toward batteries utilizing ionic liquids," said graduate student researcher Matthew Gebbie, first author of the paper, "Ionic liquids behave as dilute electrolyte solutions."
An electrolyte is a compound that is dissolved in a solution –– usually water –– in order to separate the individual, charged atoms of the compound. Take, for example, sulfuric acid dissolved in water to provide the free ions that create the charge given off by automotive batteries. Electrodes pick up the positively and negatively charged ions and deliver the current where it's needed to start the car or power electrical components.
An ionic liquid is a salt –– like rock salt but in the liquid state –– usually one that can melt at temperatures from ambient room temperatures to 100 degrees Celsius (212 degrees Fahrenheit), so the liquid is composed entirely of homogenous molecules with positive and negative charges (ions).
"You'd expect that at room temperature, with ionic liquids that are made entirely of positive and negative charges, that the ions should be mobile," said Jacob Israelachvili, professor in the Departments of Chemical Engineering and Materials.
But, despite the abundance of ions and a free-flowing environment, ionic liquids have never lived up to their promise of delivering the same kind of energy as currently available electrolytes, like sulfuric acid. Their conductivity is just not as high, said the scientists.
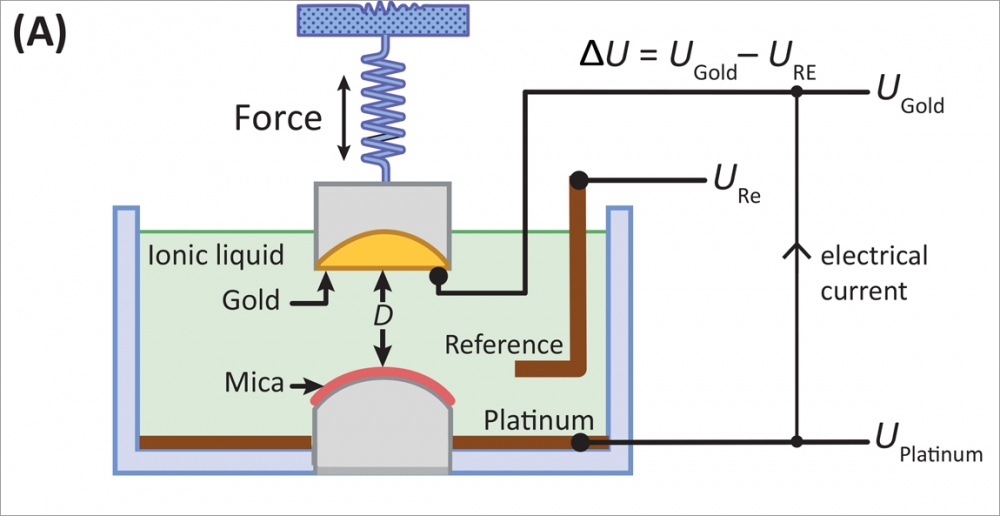
Using a surface forces apparatus, a device developed in the Israelachvili lab that can measure forces between surfaces to the sub-nano scale, the researchers analyzed the interactions of the charges in an ionic liquid –– how the surfaces attract or repel each other, the effective voltage of the liquid, and the ions' interactions with each other, as well as with the electrodes that are meant to pick up or discharge, and thereby conduct their charges.
They found that the ions in the ionic liquids are "stickier" than previously thought.
"They're bound to each other, and it's related to a complex property of any liquid or material, called the dielectric constant, which is the measure of how much you would expect charges to be free," explained Israelachvili. In fact, the somewhat-overlooked dielectric constant, which is a measure of how well charged particles stick to each other in a liquid, plays a larger role in the conductivity of ionic liquids than was previously assumed. Instead of the estimates of 50 percent separation that have been made, the experiments with the surface forces apparatus yielded a less than 0.02 percent separation between ions for typical ionic liquids.
"The connection that nobody had made before that emerged from our work was that it's not enough just to know how sticky the ions are to each other in a vacuum; you need to account for all the other billions of ions that surround any two ions in the liquid state," said Gebbie.
With that parameter taken into account along with the materials' dielectric constant, said the scientists, it became possible to come up with a simple equation that can quantitatively predict the number of free –– effectively separated –– ions that are present in ionic liquids.
"It's so simple. It really captures the physics of what's going on, but it's also simple enough to be used for predictive purposes," said Gebbie, adding that the group is now in active discussions with potential collaborators to refine and improve the equation.
The research has wide implications. With the formula, it would be possible to design an ionic liquid with particular desired properties, instead of performing countless trial-and-error tests or experiments. To date, over a million combinations of positive and negative ions have been identified that can be mixed together to form an ionic liquid, according to the researchers. To further blend these liquids to find, change, or add properties, the number of possible combinations shoots up to about 1018, or a trillion trillion potential combinations.
Not only could efficient charge-conducting ionic liquids be found in a shorter amount of time, but other properties could also be incorporated via molecular fine-tuning, such as less toxicity, reduced corrosiveness, or increased biodegradability.
"An electric vehicle has to have a very large battery. So if that very large battery is based on something that's acid, then you have a large compartment of acid. In an accident, if you had a nonflammable, nontoxic ionic liquid, then at least you could take some of that risk out of the equation," said Gebbie.
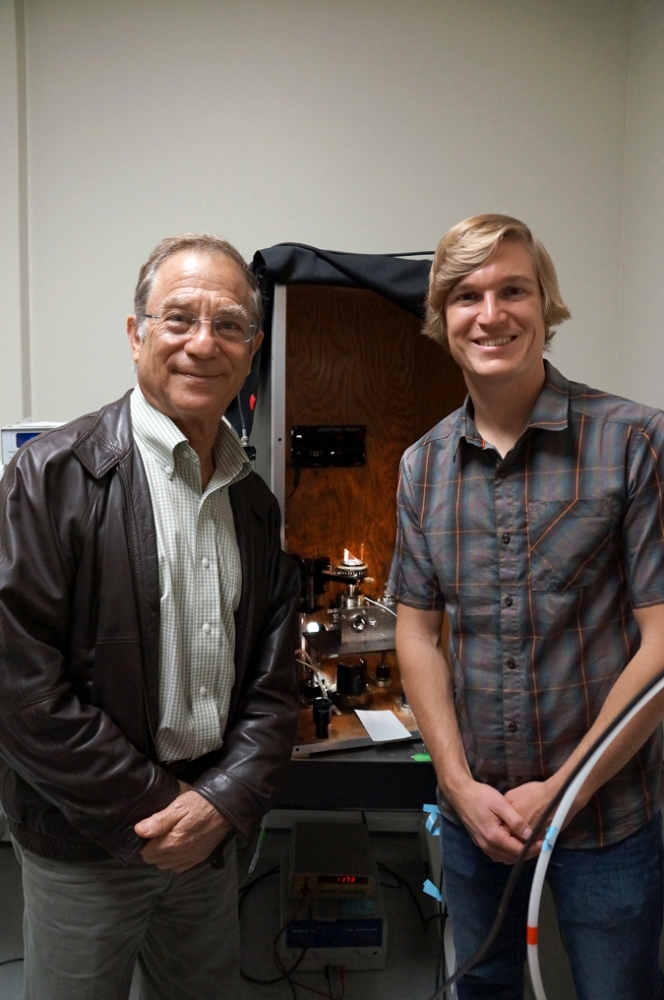
† Top image: Jacob Israelachvili, UCSB professor in the Departments of Chemical Engineering and Materials, left, and graduate student researcher Matthew Gebbie. Between them is the surface forces apparatus, used to measure the interactions between ions in ionic liquids.
Sonia Fernandez/Office of Public Affairs.
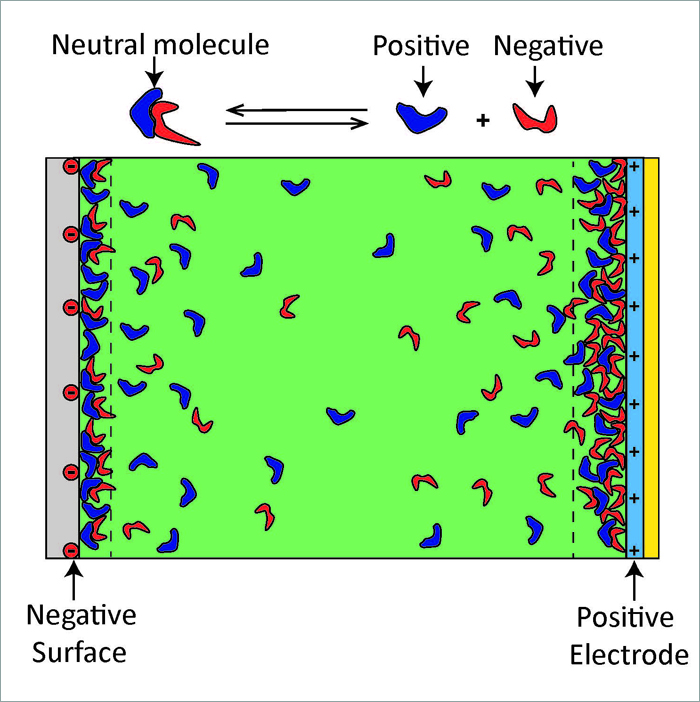
†† Bottom image: This is a cartoon depicting how the ionic liquid molecules arrange in electrically charged interfaces (not to scale). The green shading represents the 99.98% of the molecules that exist in a neutral state, the blue shapes represent positive ions and the red shapes represent negative ions. The reaction shown above the cartoon illustrates the fact that the molecules can exist in two states: a neutral ionic liquid molecule (99.98 % of the molecules) and separated positive (blue) and negative (red) ions (0.02% of the molecules).
Figures courtesy Matthew Gebbie.
Related Links
Matthew Gebbie