Marine Sponge Yields Nanoscale Secrets That May Have Hi-Tech Applications, Report UCSB Scientists
The simple marine sponge is inspiring cutting-edge research in the design of new materials at the University of California, Santa Barbara.
A report about these exciting new results involving the use of gold nanoparticles is the cover story of the current issue of the scientific journal, Advanced Materials. The article is written by Daniel E. Morse, professor of molecular, cellular and developmental biology at UCSB, and director of the Institute for Collaborative Biotechnologies, and his research group. The authors include postdoctoral fellow, David Kisailus (first author), and graduate students Mark Najarian and James C. Weaver.
The simple sponge fits into the palm of your hand, and proliferates in the ocean next to the UCSB campus, said Morse. "When you remove the tissue you're left with a handful of fiberglass needles as fine as spun glass or cotton. This primitive skeleton supports the structure of the sponge, and we've discovered how this glass is made biologically."
The newly reported research describes an important step forward in translating nature's production methods in the biological world into practical methods for the development of new materials in the laboratory.
The research team developed a method for coupling small, inexpensive synthetic molecules (that duplicate those found at the active center of the bio-catalyst of the marine sponge) onto the surfaces of gold nanoparticles. They showed that when two populations of these chemically modified nanoparticles, each bearing half of the catalytic site, are brought together, they function just as the natural biological catalyst does to make silica at low temperatures.
The UCSB scientists are already taking the next steps toward the development of practical new and useful methods of nanoscale production by incorporating catalytic components on the flat surfaces of silicon wafers, using these techniques to create nanoscale patterns of their catalyst. They are learning how to write nanoscale features of semi-conductors on these chip surfaces.
A few years ago, Morse and his research group began investigating how nature builds materials from silicon. Silicon is particularly interesting to Morse, because it is considered by many to be the most important element on the planet technologically. Silicon chips are fundamental components of computers and telecommunications devices. In combination with oxygen, silicon forms fiber optics and drives other high-tech applications.
Morse explained that his research group discovered that the center of the sponge's fine glass needles contains a filament of protein that controls the synthesis of the needles. By cloning and sequencing the DNA of the gene that codes for this protein, they found that the protein is an enzyme that acts as a catalyst---a surprising discovery. Never before had a protein been found to serve as a catalyst to promote chemical reactions to form the glass or a rock-like material of a biomineral. From that discovery, the researchers learned that this enzyme actively promotes the formation of the glass while simultaneously serving as a template to guide the shape of the growing mineral (glass) that it produces.
These discoveries are significant because they represent a low temperature, biotechnological, catalytic route to the nanostructural fabrication of valuable materials. Nature produces silica on a scale of gigatons---thousands of millions of tons---thousands-fold more than man can produce, said Morse. "This biosynthesis is remarkable because this nanoscale precision can't be duplicated by man."
Besides this remarkable precision, nature manages to produce silica at a low temperature, in an environmentally friendly way without the use of caustic chemicals, whereas man must use very high temperatures, high vacuums, and dangerous chemicals requiring costly remediation.
Although the reported research marks an important step forward, Morse believes that the use of these biological methods to control such syntheses would be impractical on an industrial scale. The high cost of the purification of these proteins, the requirement of the proteins for a watery environment, and their instability, all make their incorporation into electronic devices impractical. Furthermore, the presence of proteins would be incompatible with the high electronic performance required for today's device applications.
Instead, the scientists expect that by learning the fundamental mechanism used in nature, that mechanism could be translated into a practical and low-cost manufacturing method. Such a "biomimetic" approach will eventually be used in industry, said Morse.
This work is supported by the Department of Energy, the U.S. Army-supported Institute for Collaborative Biotechnologies at UCSB, NASA, and the Department of Commerce.
###
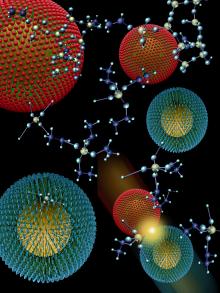
† About the Illustration
Gold nanoparticles are coated with a layer of molecules (red or green) that contribute one half of the structure required to catalyze the synthesis of silica at low temperature. These particles thus are
"biomimetic," duplicating the mechanism of synthesis discovered in a protein that catalyzes formation of the silica skeleton of a marine sponge. When the two kinds of particles collide with the appropriate chemical building block (as shown in the upper right), they catalyze the transformation that begins to link the building blocks together to form a kind of glass. When only one of these kinds of particles is present, no reaction occurs.
Applications of this environmentally benign synthesis method are anticipated in fields as diverse as microelectronics, optoelectronics, solar energy and medicine.
Related Links



