Few people have heard of the chemical ethylene oxide, but all of us have used products that rely on it for their production, from antifreeze and plastics to textiles and disinfectants. It is what’s known as a platform chemical — one that is the basis for many other chemicals — and it’s worth about $40 billion per year on the global market. But the ethylene oxide production process emits millions of tons of CO2 into the atmosphere annually, contributing to climate change. And the current process also requires chlorine, which is toxic.
Now a team of researchers, including UC Santa Barbara chemical engineering professor Phillip Christopher and Ph.D. student Anika Jalil, who is writing her dissertation on the subject, has discovered a way to potentially reduce CO2 emissions and decrease the need for chlorine to produce ethylene oxide.
Writing in the journal Science, the researchers describe how adding small amounts of nickel atoms to silver catalysts results in a reaction that is just as efficient as the chlorine-based approach but requires no chlorine. The finding could contribute to the more efficient production of this ubiquitous chemical.
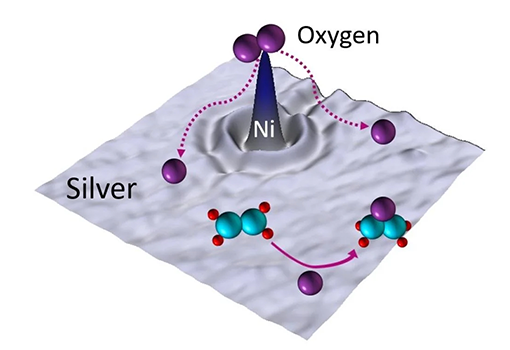
It was a long road to the discovery. Charles Sykes, a professor at Tufts University, first discussed the idea with collaborator Matthew Montemore, a chemical engineering professor at Tulane University, six years ago. They were interested in exploring selective oxidation reactions, and settled on ethylene oxide production, which is made from ethylene and molecular oxygen.
Catalysts — substances that increase the rate of chemical reactions without undergoing chemical change themselves — break the strong O2 bond, allowing single atoms of oxygen to bind with the ethylene to form ethylene oxide. Silver is the main catalyst for making ethylene oxide, but it produces two molecules of CO2 for every molecule of ethylene oxide; adding chlorine brings it to about one molecule of CO2 for every two ethylene oxide molecules produced.
Aware of the safety and environmental implications of current chlorine-based industrial production methods, Sykes and Montemore looked for elements they could add to the silver catalyst to substitute for chlorine. “The answer was nickel,” Sykes said, “which surprised us, because we couldn’t find anything in the scientific or patent literature about nickel despite its being a common and inexpensive element used in many other catalytic processes. Could seventy-plus years of industrial R&D have missed it?”
Fundamental experiments conducted at Tufts were promising. Using a single-atom alloy concept developed by Sykes more than a decade earlier, the researchers were able to discover that by adding individual atoms of nickel to silver, they could test how the material would work as a catalyst. “Our results indicated that it may be applicable to real industrial catalysts,” Sykes said.
To test it, he enlisted Christopher, the UCSB Rinker Founder's Chair and Mellichamp Chair in Sustainable Manufacturing, to make a new formulation of the silver catalyst by adding tiny amounts — individual atoms — of nickel. “Selective oxidation is one of the more challenging reactions, and so I wouldn’t have been surprised if it hadn’t worked,” said Sykes. But it did.
“Anika took on the extremely difficult technical challenge of developing a reproducible protocol for incorporating nickel atoms into the silver catalyst,” Christopher said of Jalil. “The difficulty of doing that could be why the effect we observed was never previously reported."
Jalil, a fifth-year Ph.D. student, has been working on this problem since arriving at UCSB in 2020, following her graduation from Rutgers University with a bachelor’s degree in chemical engineering. After completing her doctoral degree later this spring, she will assume a position as a senior researcher at Dow Inc.
“Someone might see this result and think it’s straightforward,” Jalil said, noting that it was anything but that. “We had a lot of failures, and for the longest time it felt impossible, then it became very hard, and then it became something that was doable. We kept working at it systematically and, ultimately, ended up with some exciting and reproducible results.
“The design phase space (the range of all possible choices and combinations you can make when designing something) in this project was massive, with many variables to take into account,” Jalil added. “We calculated a way to narrow down that space to establish the correct ratio of nickel atoms to silver atoms, and ended up using one nickel atom for every two hundred atoms of silver.”