Researchers uncover a potential method for interrupting the misfolding of tau protein that underlies neurodegenerative disease
A spectrum of neurodegenerative diseases, including frontotemporal dementia (FTD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are due to the accumulation of abnormal, misfolded tau proteins in the brain. A team of researchers led by UC Santa Barbara scientists has found potential ways to interrupt this process by targeting “sticky” sites along the long form of mutated tau, preventing the misfolding and spreading of the neurofibrillary tangles.
“This is a true collaboration of biology and chemistry,” said UCSB neuroscientist Kenneth S. Kosik, who with chemistry professors Songi Han, Joan-Emma Shea and chemical engineering professor Scott Shell, presents findings in the Proceedings of the National Academy of Sciences. The study presents molecular-level insights on the way pathological tau spreads, and according to the researchers, this understanding could lead toward “a therapeutic intervention potentially capable of disaggregating tau or preventing its aggregation” in the long form of tau accumulates.
A sticky hairpin
Tau is an essential structural protein in the brain, giving cells form and stability and allowing the transport of necessary nutrients. However, when it mutates and misfolds, it can get sticky and tangled. Moreover, this error in folding can become a template for faulty instructions that direct normal tau proteins to misfold and collect until the condition spreads over wide regions of the brain, interfering with brain functions. The specific locations where these neurofibrillary tangles occur in the brain differ among the neurodegenerative disorders.
There are two particular forms of tau, which serve as the starting point for this category of neurodegenerative diseases, called tauopathies, and for which Alzheimer’s is the most well-known. Tau is produced in both a short “three-repeat” version and a longer “four-repeat” version; the latter the focus of this research. Tauopathies far less common than Alzheimer’s such as FTD, PSP and CBD are exclusively tauopathies of the 4R type, though neurodegenerative diseases can also be associated with the 3R form, or, as in the case of Alzheimer’s, a combination of both. The discoveries in this research pertain to diseases that accumulate 4R tau.
Merging advanced techniques such as transmission electron microscopy and molecular dynamics simulations with in-vitro experiments involving cell cultures, the research team was able to get a sense of the conditions under which pathological 4R tau begins to misfold, template other tau proteins, and aggregate.
“Tau folds in a unique way in each of these diseases,” Kosik explained. “One part of it folds into a hairpin structure only in the 4R tau.” Within the hairpin, he explained, is a sticky segment called PHF6 that can bind and stack up other tau proteins into large aggregations.
But what if it were possible to induce tau aggregation in cell culture and use the system to interfere with this sticky site? “Creating conditions for tau propagation serves as a high throughput system for the discovery of compounds that may interfere with tau aggregation,” Kosik commented. They found, for instance, that a single amino acid substitution along the protein adjacent to the sticky region was enough to prevent tau aggregation by weakening access to the vulnerable portion of the peptide.
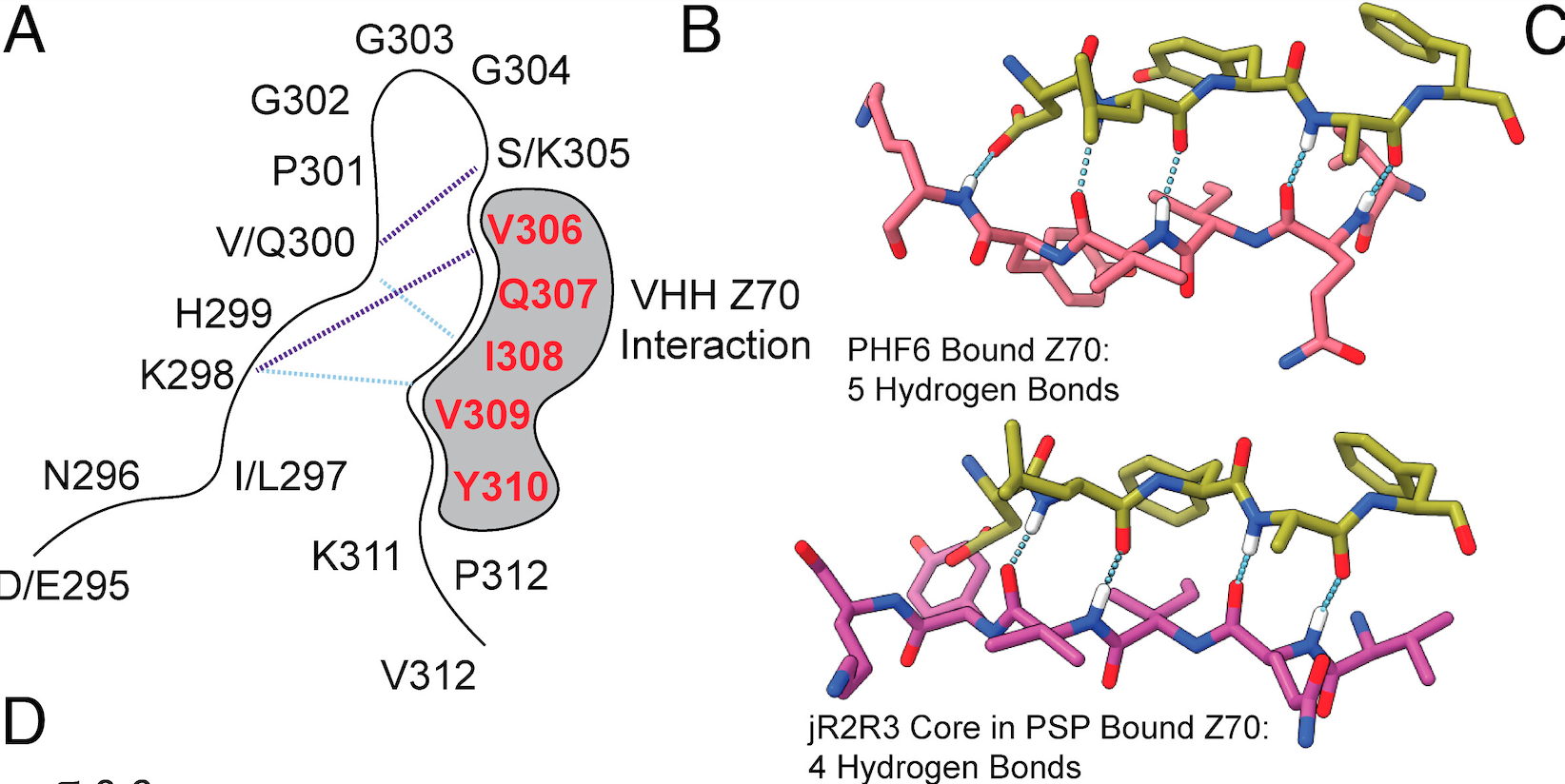
In other investigations the researchers also found that nanobodies — fragments of antibodies — synthesized from the blood of camelids (camels, llamas and other members of the Camelidae family) were able to bind to the PHF6 region, inhibiting aggregation of tau. Whatever the therapy, the region encompassing the hairpin segment of 4R tau is the apparent active zone to target.
The researchers still have a long way before targeted therapeutics can be developed and approved to inhibit the formation of the neurofibrillary tangles that characterize tauopathies. But the findings in this paper unveil exciting potential pathways to arresting critical steps toward the accumulation of mutant tau.
“We would want to continue to test this technology in animal models,” Kosik said, crediting postdoctoral researcher and lead author Andrew Longhini for his “enormous contributions” to ideas and the experiments reported. On the chemistry side, the work of graduate student researchers Austin DeBose and Samuel
Lobo in engineering protein interactions and doing the computational heavy lifting was also essential to the project, he said.
“It’s so interdisciplinary, it’s amazing,” Kosik said. “I learned so much from this project.” In addition to advancing research on some forms of neurodegenerative disease, these findings can serve as a jumping-off point for potential clues toward therapies for the 3R Pick’s disease and for the more complex Alzheimer’s disease. Kosik is optimistic.
“We’ll try to apply our findings to the more complicated forms,” he said. “We’ll get there.”
Research in this project was supported by the Keck Foundation.
Sonia Fernandez
Senior Science Writer
(805) 893-4765
sonia.fernandez@ucsb.edu