Shuffling the Deck


We all must play the game of life with the cards we’re dealt, so the common aphorism goes. In biology, this means organisms must compete through natural selection with the genes and anatomy they were born with.
But the saying is a lie.
Okay, it’s not exactly a lie, but modern research suggests that the game of life is far more complicated than we had anticipated. There are opportunities to swap cards and even steal other players’ hands.
Researchers at UC Santa Barbara have been investigating the effects of this strategy, particularly the ability to acquire metabolic pathways. The scientists found that adopting another metabolism can have major competitive consequences, with ramifications to a species’ evolution and ecology. The results appear in the journal Ecology.
The term “metabolism” encompasses all the chemical reactions that take place in an organism in order to maintain life. For animals, this includes the nuts and bolts of processes like respiration, digestion, movement, etc. An acquired metabolism is a metabolic pathway that is not encoded in an organism’s DNA.
Examples of acquired metabolisms abound in nature. Some are familiar, like the microbes in a cow’s gut that enable it to digest cellulose. Others are more common but less well-known. For instance, consider the symbiotic fungi that help plants source minerals from the soil. And then there are truly unusual acquired metabolisms, like sea slugs that steal chloroplasts from their food so they can photosynthesize.
While acquired metabolisms are well-attested in the literature, previous research mostly considered its interactions with environmental factors. The UC Santa Barbara group investigated their role in growth and community dynamics, focusing on acquired phototrophy, like that of the sea slug. “We really wanted to understand whether or not this acquired phototrophy would give an organism a competitive advantage,” said lead author Veronica Hsu, who completed the study as an undergraduate.
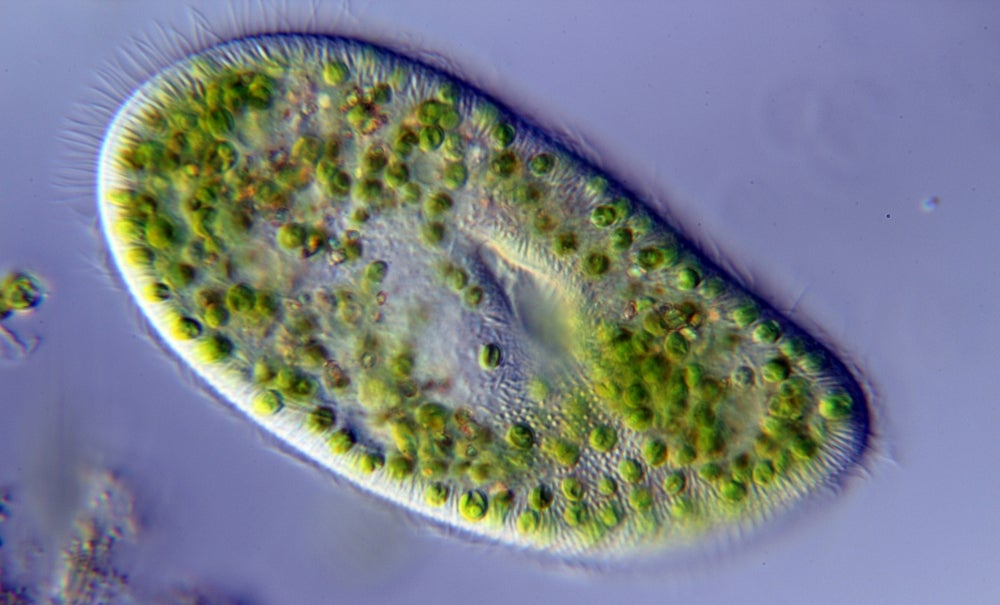
The authors considered two single-celled eukaryotes (organisms whose cells contain a nucleus). The first, a species in the genus Colpidium, subsists on a diet of smaller microbes. The second, Paramecium bursaria, shares its counterpart’s diet, but had also acquired the ability to photosynthesize at some point in the past.
The researchers analyzed the two microbes under four different light conditions. Colpidium got along fine no matter the setting; however, P. bursaria fared much better under brighter conditions, where it could take advantage of its unique ability.
Then the scientists pitted the microbes against each other. They observed a gradient of competitive advantage across different light levels. In the dark, Colpidium outcompeted P. bursaria. Meanwhile, under bright conditions, P. bursaria dominated.
“I think it gets to this idea that you can’t be good at everything,” said co-author Holly Moeller, an assistant professor in the Department of Ecology, Evolution and Marine Biology. Adapting to an acquired metabolism might have come at the expense of P. bursaria’s hunting prowess. But at high light levels, the boost from photosynthesis more than offsets this handicap.
Remarkably, the two microbes were able to coexist under intermediate light conditions. P. bursaria’s acquired phototrophy enabled it to avoid direct competition with Colpidium in what scientists call “niche partitioning.”
The results demonstrate that symbiosis and acquired metabolism can drastically affect community dynamics. “Expanding on your metabolic repertoire has cascading implications on how you can make a living, and the extent to which you’re going to shove other organisms out of the way,” Moeller said.
The researchers then turned to the trusty Lotka-Volterra model to describe what they had witnessed. This model is incredibly simple and versatile, providing biologists a system that can capture all the possible outcomes of competition. Developed over 100 years ago, it has become a go-to standard for intro biology classes all the way to peer-reviewed research.
And yet, this stalwart system couldn’t capture the subtlety introduced by P. bursaria’s acquired phototrophy and the feedback cycle it created. The team had to develop their own system of equations that explicitly accounted for these nuances. “There are a lot of different ways to try to explain competitive outcomes,” Hsu said, “and I think this shines a light on how important metabolism can be.”
It’s important to study how acquired metabolisms influence evolution and ecology because they’re a fundamental part of life on Earth. For instance, we generally think of photosynthesis as a characteristic of plants. “But that’s an ancient acquisition, too,” said Moeller. “They inherited their chloroplasts from a eukaryotic ancestor that domesticated a cyanobacterium.”
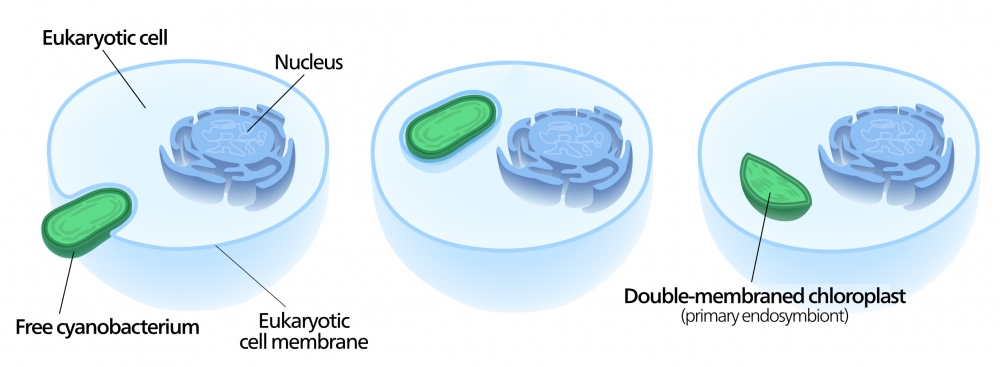
Many scientists believe that an ancestor of plants once caught a cyanobacterium, but rather than digest it, the cell kept its acquisition around. Eventually, the bacteria evolved into an organelle: the chloroplast.
Photo Credit: KELVIN13 VIA WIKIMEDIA
“Mitochondria are also acquired from bacteria,” added Hsu. In fact, both of these organelles have their own DNA, separate from a cell’s nuclear genome.
“This is how eukaryotes have been playing the game for some 2 billion years,” Moeller remarked. And our simpler counterparts, prokaryotes, arguably engage in even more biological card-swapping. Many are able to directly share DNA in a process known as “horizontal gene transfer.”
Moeller’s group will continue to study the implications of acquired metabolisms. They’re particularly curious about the transition from heterotrophy (obtaining food externally) to autotrophy (producing food oneself), especially photosynthesis. “We’re trying to understand what causes these forms of metabolism to jump around the tips of the branches of the tree of life,” she said.
Moeller plans to use mathematic models to investigate these transitions in addition to looking for real-world case studies. And future experiments will involve microbes that are more closely related to each other, enabling the team to control more variables. “The experiments help us build better models,” she said, “while the models help us better understand what happened in the experiments.”
More research is certainly welcome. Because, in this convoluted corner of biology, at least one thing has become abundantly clear: We’d have a less dynamic, less complex ecology on this planet if organisms could play only with the cards they were dealt.



