Zooming In

The microwave oven has been around for almost 80 years. When it heats food or liquid, the frequency of electrons increases but their energy slows down due to their own microwave emissions. Until now, scientists have only been able to observe this phenomenon in a group of electrons.
However, Project 8, a collaboration of 27 scientists from six institutions in the United States and Germany, has for the first time been able to detect the frequency of radiation emitted by an individual, orbiting electron. The group’s findings appear in the journal Physical Review Letters.
“One of the reasons our result is exciting is that it gives us a new way of capturing electrons to use as a back door into studying neutrinos,” said Benjamin Monreal, an assistant professor in the Department of Physics. “We hope it will lead to a measurement of the neutrino mass, which is currently one of the last remaining unknowns in the Standard Model of particle physics.”
The second-most abundant particles in the universe, neutrinos lack an electric charge and are produced by the decay of radioactive elements. They come in three varieties: electron, muon and tau, each with a different, still-unknown mass. While the differences between the mass of each type of neutrino can be calculated, scientists at this point in time only know the range into which measurement of these masses will fall. Once refined, the technique developed by Project 8 has the potential to make the first direct measurement of the mass of the neutrino.
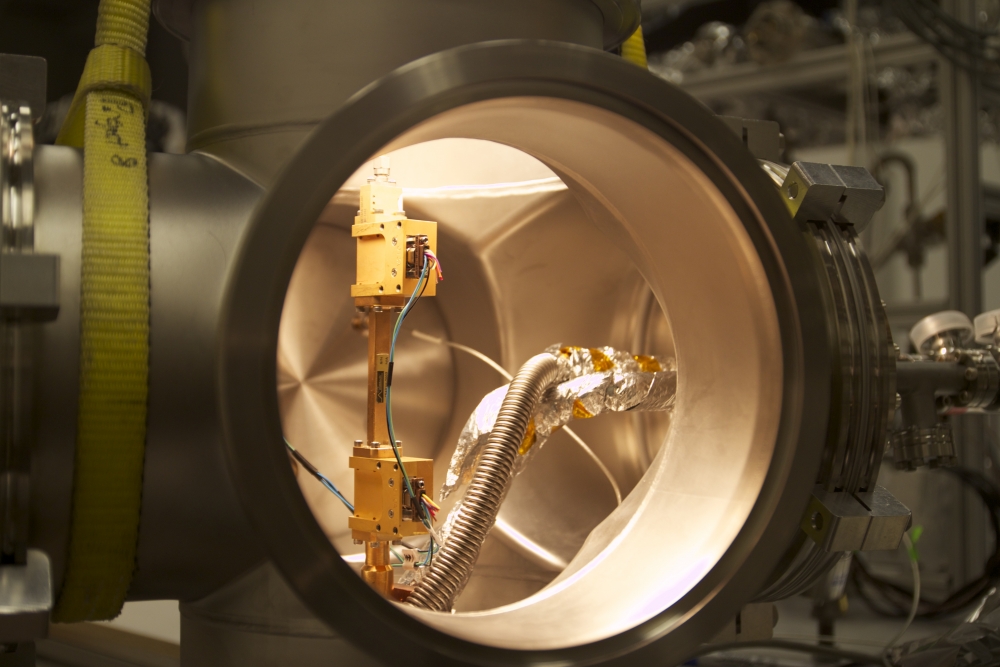
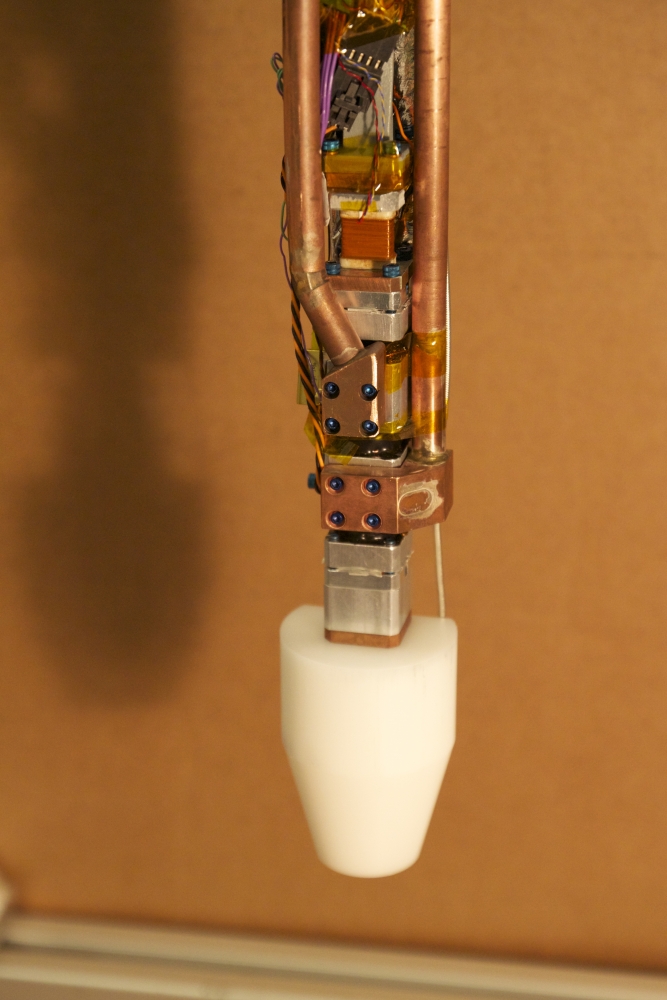
Previous electron detection and energy measurements required enormous spectrometers to measure radiation. Project 8 collaborators may have changed that. Not only were they able to detect emissions from a single electron but they did so using a tabletop instrument.
The team built a small apparatus to contain a single high-energy electron in a magnetic field containing krypton-83, a radioactive isotope that produces electrons as its nuclei undergo beta decay. Electrons from the radioactive decay move extremely fast, at 20 percent of the speed of light, and spiral in a magnetic field. Each electron emits a signal that can be measured very accurately using radio waves.
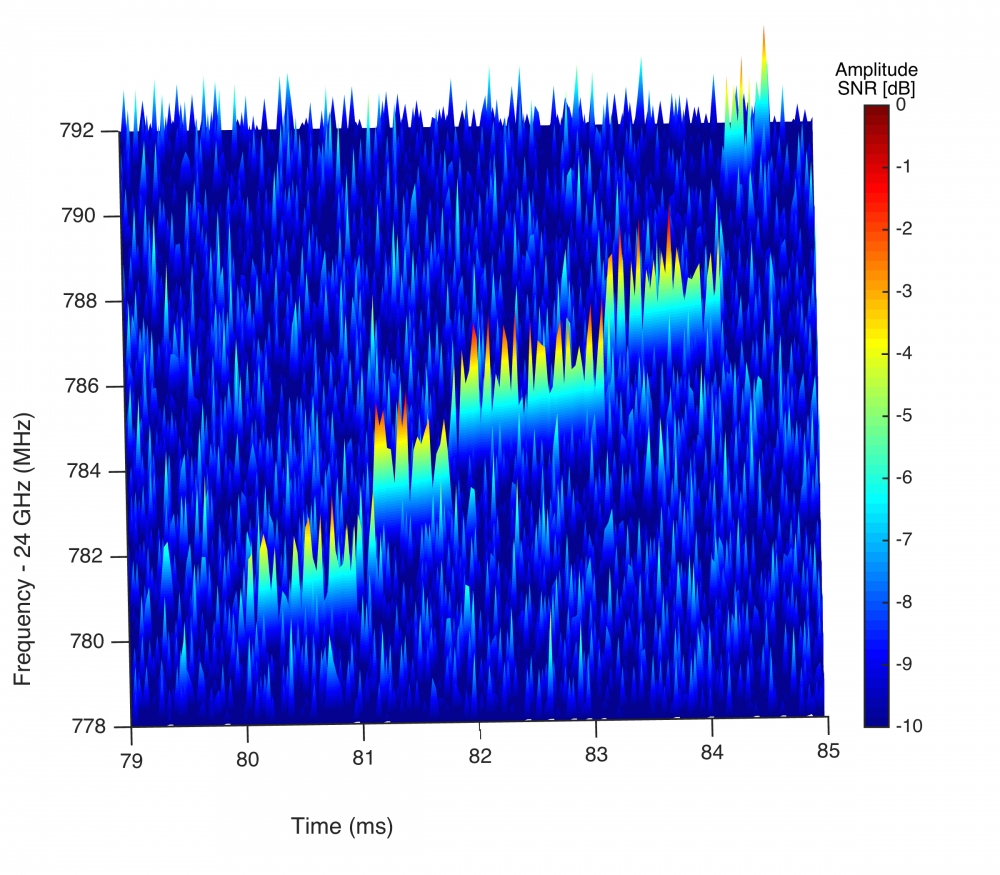
Called cyclotron radiation, this effect was predicted more than 100 years ago but has only now been observed one electron at a time. In fact, the team was able to witness the activity of more than 100,000 single electrons.
“We were able to trap an electron for about 10 milliseconds, which doesn’t sound like very long,” Monreal said, “but it’s actually taking a little 30-kilometer journey going around and around in circles. Nobody’s ever been able to zoom in on a single electron before.”
When the electron bumps into a gas molecule, it jumps and loses a fraction of its energy, which in turn increases its frequency. This sequence of events produces a characteristic chirp, which can be seen when frequency is plotted against time.
“We were able to take a single electron and see it scatter 20 times and measure every little energy change,” Monreal said. “Sometimes we could see it changing directions slightly.”
According to Monreal, Project 8 has found a new use of basic electromagnetism. The equation used by the investigators was first published in 1904. “It’s very, very old electromagnetism that we’re just pushing to the edge of the smallest charge that you can see with it,” Monreal explained.
“We have a new tool for studying radioactive decays,” he added. “For the future, the decay we’re most interested in is tritium, which is a radioactive isotope of hydrogen. Every time it decays, it emits an electron and a neutrino and you can detect those electrons. One day our instrument will be able to measure those electrons. If you can measure electron distribution precisely enough, you can figure out neutrino mass, which we’ve been talking about for 80 years now.”
Measuring neutrino mass may be the ultimate goal of Project 8, but the team’s method also has the potential to be used for environmental monitoring of nuclear fuel.
This research was funded in part by the U.S. Department of Energy and the National Science Foundation.