In a potential game changer for the treatment of superbugs, a new class of antibiotics was developed that cured mice infected with bacteria deemed nearly “untreatable” in humans — and resistance to the drug was virtually undetectable.
Developed by a research team of UC Santa Barbara scientists, the study was published in the journal eBioMedicine. The drug works by disrupting many bacterial functions simultaneously — which may explain how it killed every pathogen tested and why low-level of bacterial resistance was observed after prolonged drug exposure.
The project was led by professors Michael Mahan, David Low, Chuck Samuel and their research team, Douglas Heithoff, Scott Mahan, Lucien Barnes and Cyril George. Additional contributors include professors Guillermo Bazan (UC Santa Barbara) and Andrei Osterman (Sanford Burnham Prebys Medical Discovery Institute).
The discovery was serendipitous. The U.S. Army had a pressing need to charge cell phones while in the field — essential for soldier survival. Because bacteria are miniature power plants, compounds were designed by Bazan’s group to harness bacterial energy as a “microbial” battery. Later the idea arose to re-purpose these compounds as potential antibiotics.
“When asked to determine if the chemical compounds could serve as antibiotics, we thought they would be highly toxic to human cells similar to bleach,” said Mahan, the project lead. “Most were toxic — but one was not — and it could kill every bacterial pathogen we tested.”
What makes the drug unique is the failure of bacteria to become resistant to it. And bacterial resistance is typically a major barrier to antibiotic development since it limits a drug’s (potential value in the marketplace.
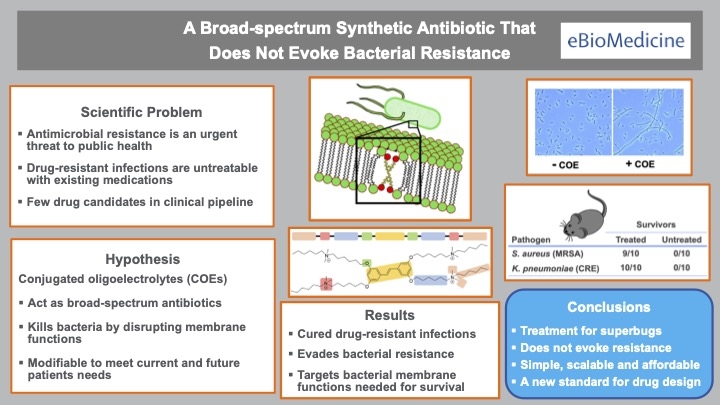
“The key finding was that bacterial resistance to the drug was virtually undetectable,” said lead author Heithoff. “Most drugs fail at this stage of development and never get to clinical practice.”
The antibiotic has a unique mechanism of action. Contrary to most drugs (like penicillin) that target a specific germ function, the new drug targets many functions simultaneously.
“The drug appears to affect the bacterial membrane which, in turn, disrupts multiple bacterial functions,” explained Low, the co-project lead. “This may account for the broad-spectrum antibacterial activity and low level of bacterial resistance.”
“This class of antibiotics has potential as a new versatile therapy for antimicrobial resistant pathogens,” Samuel said.
Additional drug safety and efficacy studies will need to be conducted to fully understand the clinical benefits and risks before the drug can be used in clinical practice.
The study, “A Broad-spectrum Synthetic Antibiotic That Does Not Evoke Bacterial Resistance,” was supported by grants from the National Institutes of Health, and the U.S. Army Research Office via the Institute for Collaborative Biotechnologies (ICB) cooperative agreement and contract. The research was funded via a $4 million Congressional addition that Representative Salud Carbajal was able to add to the FY20 Defense Appropriations Bill for the UC Santa Barbara ICB.
“UC Santa Barbara has always been a leader in research, technology and development," Carbajal remarked. "The finding of this new class of antibiotics reaffirms the talent we have in the Central Coast, and marks a big step forward in our country's biotechnology and national defense.
"As a Gaucho myself, I'm proud to have helped secure the additional funding that has supported the researchers and scientists on our campus and enabled them to continue making incredible discoveries like this one, and I will continue advocating for more resources to enhance our educational partnership programs.”
Shelly Leachman
(805) 893-4620
shellyleachman@ucsb.edu