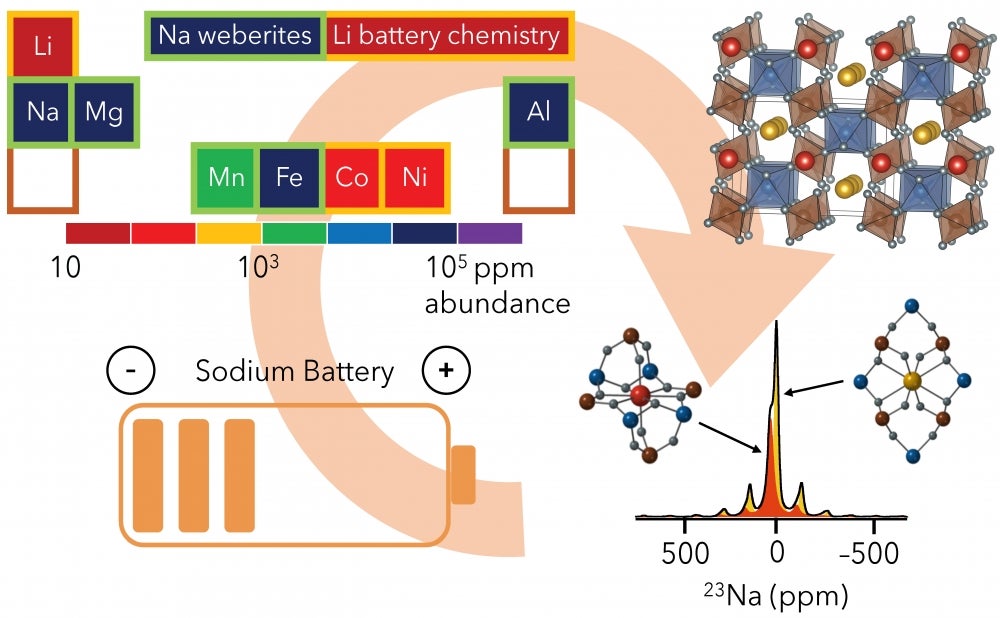
The Sodium Solution

Lithium (Li) is the active ingredient in rechargeable batteries that power today’s smart phones, laptops and electric vehicles. However, lithium’s scarcity and high price have led researchers to seek another, more abundant element to replace it. Many have turned to sodium (Na), which is below lithium in the periodic table and shares many of its properties. Sodium is nearly 1,200 times more available in the world than lithium, and its abundance makes it more affordable to extract and purify.
The deployment of Na-ion batteries, which function through reversible insertion and extraction of Na into/from the electrode material, has been hampered by the lack of cathode materials that are capable of storing large amounts of charge reversibly.
“Viable sodium alternatives to current lithium-based batteries have proven elusive, partly because a limited number of sodium-ion electrode materials have been tested to date,” said Raphaële Clément, an assistant professor in UC Santa Barbara’s Materials Department.
To find new sodium cathode materials, Clément proposes to study derivatives of Na2MgAlF7, a new class of high energy density compounds comprising Earth-abundant elements like fluorine, sodium, magnesium, aluminum, manganese and iron. The weberite mineral radically departs from systems that have already been explored for lithium, and prior research has shown that transition metal fluorides could be promising cathode materials for sodium.
In support of her novel research, Clément has received the Early CAREER Award, the most prestigious award given by the National Science Foundation (NSF) to support early-career faculty. As part of NSF’s CAREER program, Clément will receive more than $700,000 over five years to fund her proposed research and educational activities.
“We are extremely proud of Raphaële Clément, and we congratulate her on receiving this esteemed award,” said Tresa Pollock, interim dean of UCSB’s College of Engineering (COE) and Alcoa Distinguished Professor of Materials. “She is a shining example of the high-quality junior faculty we have in the college, who seek to create new knowledge and innovations that address grand challenges, such as energy efficiency and sustainability.”
“I am thrilled about this opportunity to establish a long-term research program on topics that I am passionate about, to engage with the broader public and colleagues on issues related to climate change and climate justice, and to contribute to the materials advances needed to develop the next generation of low-cost, sustainable and energy-dense rechargeable batteries,” said Clément, who joined UCSB in 2018 after earning her Ph.D. in chemistry from the University of Cambridge. “I am grateful for having a fantastic group of undergraduate and graduate students and postdoctoral researchers. I would not have received this award without their diligent work and their enthusiastic approach to outreach and education.”
As part of Clément's project, “High-Resolution NMR for Paramagnetic Sodium Electrodes,” researchers in her lab will explore the new materials at a fundamental level, seeking to understand the links between their chemistry, atomic structure and electrochemical performance. They plan to achieve this, she explained, by using nuclear magnetic resonance (NMR) spectroscopy, a powerful technique that makes it possible to analyze the atomic structure of a material by tracking interactions between nuclear and electronic spins (tiny bar magnets associated with atomic nuclei and electrons) when the material is placed in a powerful magnetic field. NMR allows scientists to study the charge-discharge processes in battery materials.
“We plan to establish a novel and unconventional NMR method to gain atomic-level insights into the working principles of these battery electrodes,” Clément said, adding that her team wants to monitor minute changes in the crystal and electronic structure. “This understanding will then be used to design new materials and chemistries with enhanced properties.”
The first step will be to determine which electrode chemistries can be prepared in the laboratory using computer simulations. They will then prepare these compositions and use NMR and other techniques to study the role that the crystal and electronic structures play in determining Na-ion diffusion, electronic conductivity and phase stability. Lastly, they will develop a novel theoretical method to predict the NMR properties of complex battery electrode materials.
Clément says that the battery chemistries targeted in this project could have significant societal impacts as they altogether eliminate issues of toxicity, raw materials supply and cost that plague current Li-ion batteries.
“These materials hold promise for high-energy density and high-power battery applications and could lead to the discovery of potentially transformative chemistries for large-scale energy storage,” she said.
Clément is the tenth junior faculty member from the UCSB College of Engineering since April 2020 to receive a CAREER award. UCSB ranks first among public universities in the percentage of eligible-junior faculty who received the awards from 2017-2021.



