The Body’s Transformers



Like the shape-shifting robots of “Transformers” fame, a unique class of proteins in the human body also has the ability to alter their configuration. These so-named intrinsically disordered proteins (IDPs) lack a fixed or ordered three-dimensional structure, which can be influenced by exposure to various chemicals and cellular modifications.
A new study by a team of UC Santa Barbara scientists looked at a particular IDP called tau, which plays a critical role in human physiology. Abundant in neurons located in the nervous system, tau stabilizes microtubules, the cytoskeletal elements essential for neuronal functions such as intracellular transport. Lacking a fixed 3-D structure, tau can change shape so that it forms clumps or aggregates, which are associated with Alzheimer’s disease and related dementias. The researchers’ findings appear online in the Proceedings of the National Academy of Sciences.
“In the brain, these proteins need to change shape very rapidly to adapt to different conditions,” said co-author Joan-Emma Shea, a professor in UCSB’s Department of Chemistry and Biochemistry. “It’s important to understand the relationship between protein shape and function and how can you change the shape. So we used these external agents, small molecules called osmolytes, to affect the shape or conformations of these proteins.”
The researchers not only conducted biological experiments but also ran computer simulations to understand how these small molecules change the shape of tau and, when they do so, how it affects the protein’s ability to aggregate. They found that tau’s structure — whether extended or compact — was associated with how easily it bound to other tau proteins to promote the aggregation process.
“Continual aggregation of tau can, over time, result in an accumulation of pathological aggregates known as neurofibrillary tangles,” said lead author Zachary Levine, a postdoctoral scholar in the Department of Chemistry and Biochemistry and Department of Physics. These tangles have long been known to be associated with Alzheimer’s disease and related dementias.
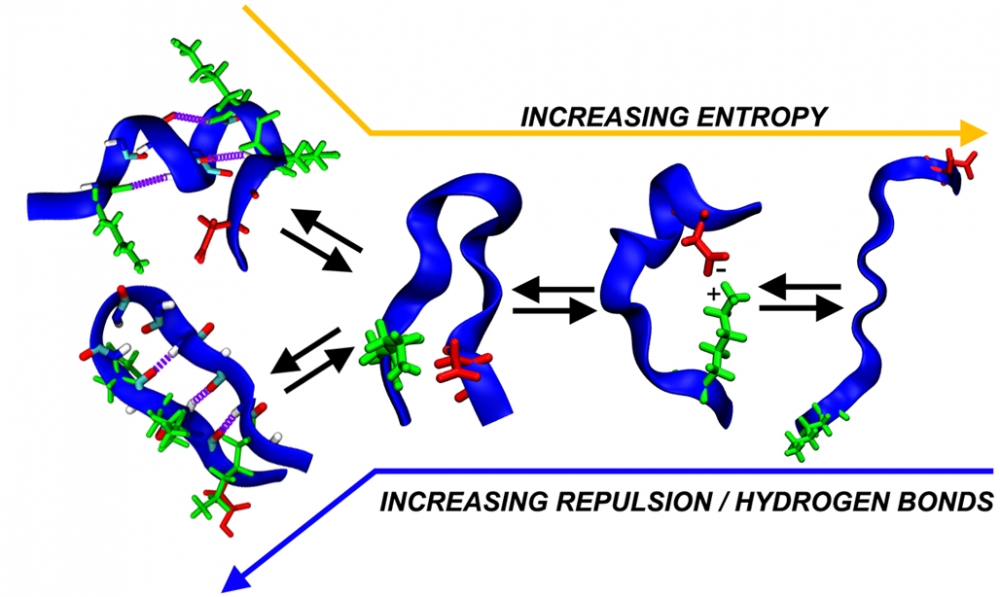
“In our computer simulations, we looked at certain chemical interactions called hydrogen bonds,” Levine explained. “We found that IDPs containing a large number of hydrogen bonds tended to take on smaller, more compact structures, but when we chemically removed them, we could create more extended protein structures. This allowed us to fine-tune what conformations tau could adopt. That’s really attractive if, say, you wanted to design a drug that intervenes in the pathological folding of IDPs.”
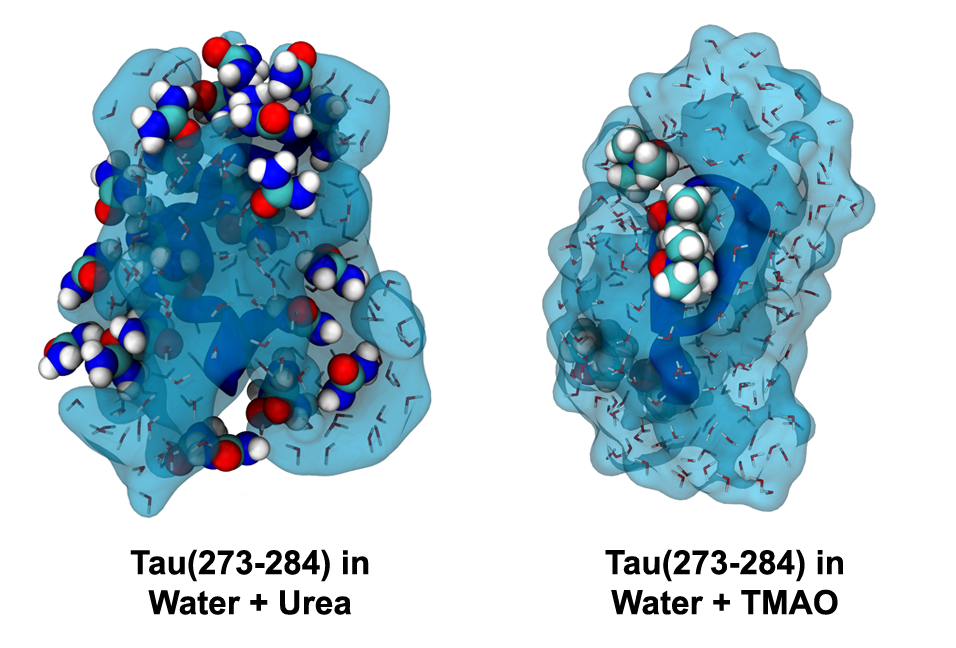
In order for aggregates of tau to develop, extended forms of the protein must stick together in a long sheet. The researchers exposed tau fragments to the osmolyte urea, which binds to the extended structures and prevents two of them from coming together. They were able to show that urea stopped aggregation, but the reason it did so wasn’t clear. However, subsequent computer simulations were able to reveal the interaction of urea with the tau protein, exposing underlying chemical interactions that decreased the likelihood of protein aggregation.
“The chemical structure of urea is quite similar to the backbone of tau,” Levine said. “Urea mimics the protein structure and binds to the surface, stopping other pieces of tau from binding to one another because the binding sites are already occupied by urea.”
The scientists also experimented with another osmolyte, trimethylamine N-oxide (TMAO), which had the exact opposite effect. They found that TMAO-exposed tau formed helical structures, which have been shown in other studies to accelerate aggregation.
“With TMAO, you can see fibrils form, but with urea you don’t,” said co-author Nichole LaPointe, a researcher in the Department of Molecular, Cellular and Developmental Biology (MCDB) and Neuroscience Research Institute (NRI) who conducted the experiments described in the paper. Fibrils are the beginning stages of harmful clumps or aggregates of tau. “So the predictions from the simulations are actually carrying forward into something pathologically relevant, which is the formation of these big aggregates.”
Only in recent years has technology advanced to the point where it can shed light on such microcellular functions. One of the interesting things to come out of this paper, according to co-author Stuart Feinstein, an MCDB professor and a co-director of the NRI, is the idea that various normal and pathological regulatory mechanisms alter the percentage of time proteins are spending in any one of these structures.
“The other great thing that comes out of this sort of collaborative research is the training of young students,” said Feinstein. “In bioengineering, physics or physical chemistry, you have a lot of established people who are trying to learn biology; on the other hand, there are a lot of established biologists who are trying to learn the more physical sciences, but in both cases, it is a retrofit. The people who intuitively understand both worlds are those trained in both disciplines in their 20s and 30s. And that — along with good science — is what comes out of a collaboration like this.”