CHEMISTS DESCRIBE "ZIPPER TEETH" OF DNA MOLECULES -- PUBLISH RESULTS IN THE JOURNAL 'NATURE'
Experimental scientists have just published findings that represent a major step forward in their understanding of the individual molecules that comprise our DNA -- the neatly coded spiral strands of information that hold all of our biological information, including whether we have red hair or black, blue eyes or brown.
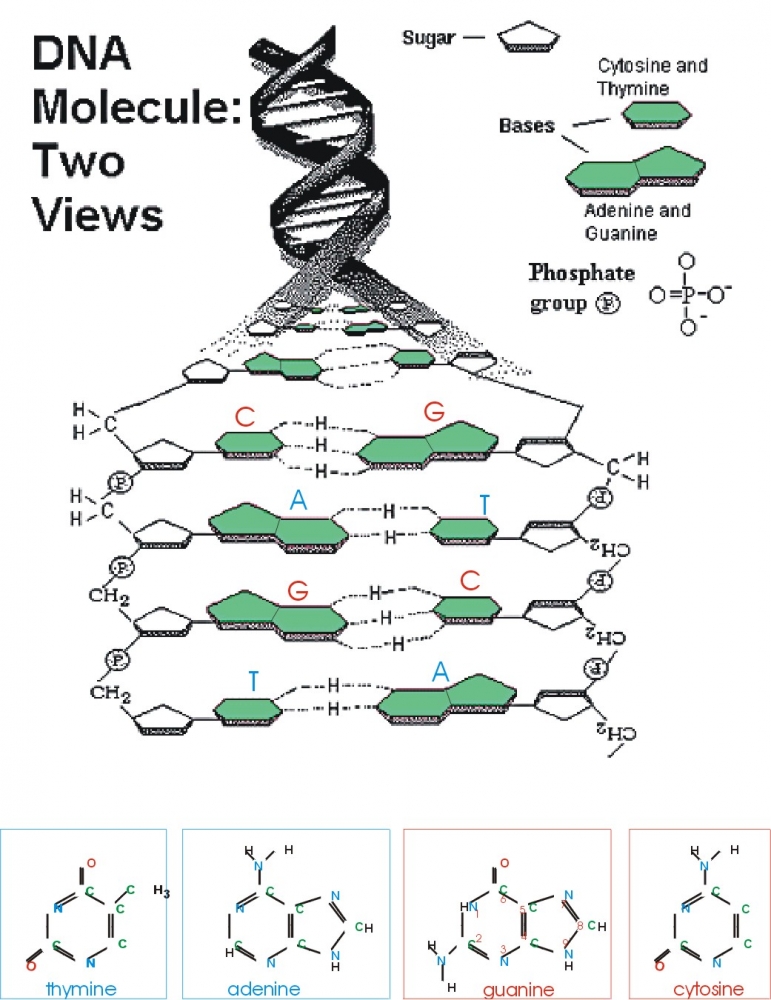
The nucleic-acid base pairs, or building blocks of DNA, have been isolated in experiments that are the first to keep pace with the rapidly growing theory of DNA's structure, researchers reported in the Dec. 21 issue of ÔNature,' the international science weekly.
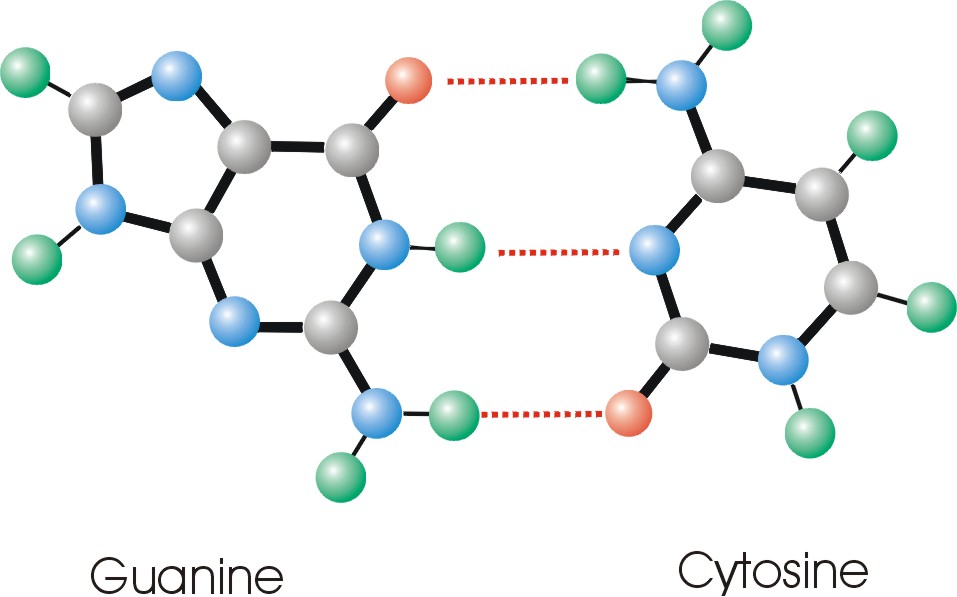
Chemists from UC Santa Barbara, California, Israel and Germany were able to "see" and thus describe the "teeth" of what they call the "zipper" that forms the double helix of DNA, the basic material of life. The article, "Pairing of Isolated Nucleic-acid bases in the Absence of the DNA Backbone," describes their discovery.
"The coding of DNA is in the order of the base pairs," said Mattanjah S. de Vries, professor of chemistry at UC Santa Barbara. "It's the secret of life. And, the heart of the mechanism is in the pairing of the base molecules. As the DNA is unzipped you get replication; the new part looks like the old part. You have to understand the structure at the level of these basic building blocks to understand how the larger structure stays together the way it does."
He explained that four molecular bases are like individual teeth of the zipper, and that his research is focused on how they come apart, or the mechanism of the zipper. In nature, the bases guanine and cytosine are always paired, as are adenine and thymine.
"I also like to explain this using Legos building blocks," he said. "When I look at a material I want to know what it does and what holds it together. To do that you have to look at the individual blocks. In the 1950s Legos patented the building blocks with 'dimples' on the top and tubes on the bottom. Without those details the entire building could not exist. The holding together of base pairs of DNA is similar to that connection.
"We would like to be able to pick up one of the individual base molecules with a pair of tweezers and hold it up to the light," said de Vries. "But we can never do that; they are too small. We have done the next best thing, we have isolated the molecules so that there is no interaction with anything else.
"To discover what part of what's going on is built into the molecule and what is due to other factors, we must first remove the other factors. We used a vacuum chamber so that not even air molecules can influence them. This is the first time that scientists have been able to look at individual base pairs. From this we can already say that there is just one way that they bond."
The researchers were also able to see the frequencies with which the bonds between the molecules vibrate, also something that has never been observed directly before.
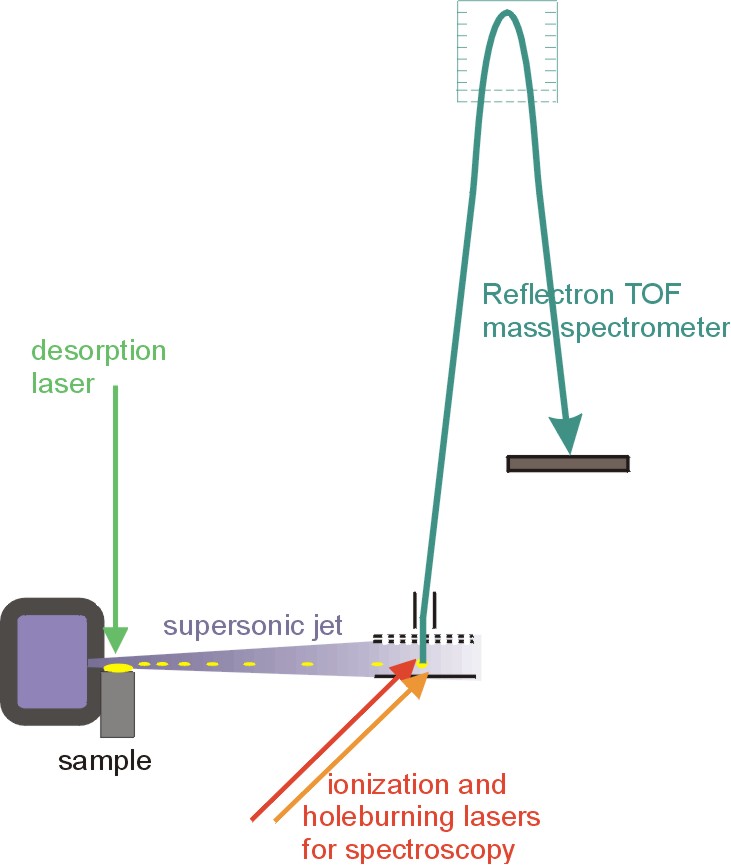
To perform the experiments, they used equipment about the size of a living room. They directed a short laser pulse (ten nanoseconds, or ten billionths of a second long) and thus were able to get the molecules free. Then they squirted a cooling gas on them and, as they cooled, found that they formed clusters of two, in mixtures of guanine and cytosine.
"At this point, these base pairs of guanine and cytosine were not interacting with anything else, no solvent, for example," said de Vries. "They are not in a cell, and are not even part of a larger DNA structure."
Then they hit the molecules with more lasers causing them to ionize. Next, they used spectroscopy, analysis of light shining through them to characterize the molecules.
"We wanted to know how pairs bind together," said de Vries. "So we took away everything, we simplified."
Watson-Crick pairing, or guanine to cytosine, is the way the structure always occurs in DNA. By taking the molecules out of the DNA, the researchers found that there is only one way that they bond. They are not quite ready to say it is the Watson-Crick pairing, but rather that it seems likely.
Regarding the work, De Vries commented, "Biology usually looks at an entire system, while chemistry takes it all apart. We want to know not just what it does, but how it does it."
He explained that the experiments included new understanding about "how things fall apart."
In some cases they saw how hydrogen (which connects the bases) ended up on the wrong part of the base pair. "This is how mistakes are introduced," said de Vries. "The fact that we can see it occur at the level of individual molecules is a major finding."
In the experiments, the researchers also looked at the bases with water molecules attached, from one to 50 molecules, thus simulating a solvent environment.
De Vries explained that theorists have been learning to do computations at a level that can't be compared to experiment. "We can now do this as a test for some of these theories," he said.
He explained that this endeavor, the study of biologically-relevant materials in the gas phase, is a new field. "The first conference on this occurred in France this year, it's a clear indication that this is an emerging field," he said.
Editors:
De Vries can be reached by telephone in Israel at:
972-2-6586109 (office) and +972-53-932210 (cell) and +972-2-6525037 (fax) or by e-mail at devries@chem.ucsb.edu. He will be back in Santa Barbara on Dec. 31.